A transition metal-free approach towards the regioselective synthesis of β-carboline tethered pyrroles and 2,3-dihydro-1H-pyrroles†
Abstract
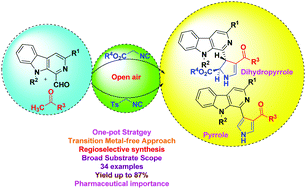
An efficient metal-free approach has been devised for the synthesis of novel β-carboline C-1 tethered 2,3-dihydropyrroles and pyrroles via one-pot assembly involving 1-formyl β-carbolines, aryl methyl ketones, and isonitriles. The reaction advances through the formation of β-carboline linked enones followed by [3+2] cycloaddition with isonitriles. This methodology features an operationally simple procedure, multicomponent character, high atom economy, a broad substrate scope, short reaction times, and high regioselectivity with excellent yields. The scope of the process was exemplified via the synthesis of a library of 34 novel compounds with significant diversity.


 Please wait while we load your content...
Please wait while we load your content...