Self-supported beaded Au@Co3O4 nanowire arrays perform electrocatalytic CO2 reduction in water to syngas and water oxidation to O2†
Abstract
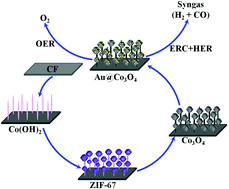
Syngas, as a necessary feedstock dependent on non-renewable fossil fuels, is a mixture of CO and H2, which could be prepared by a renewable strategy via electrochemical CO2 reduction in water electrolyte solutions. Herein, beaded Au@Co3O4 nanowire arrays (denoted as Au@Co3O4 NAs) were obtained by immersion in HAuCl4 solution with Co3O4 nanowire arrays. The Au@Co3O4 NAs exhibited outstanding electrochemical performance of producing syngas via tuning from 1 : 1 to 4 : 1 with controlling voltage. In addition, the Au@Co3O4 NAs showed better catalytic performance for the oxygen evolution reaction with an overpotential of 320 mV at a current density of 50 mA cm−2, along with a small Tafel slope of 75 mV dec−1 in 1 M KOH solution. The electrochemical performance of Au@Co3O4 NAs is obviously superior to Co3O4 NAs, which will open up a viable strategy for the design and fabrication of a bi-functional electrocatalyst for the overall reaction (CO2 + H2O → CO + H2 + O2).


 Please wait while we load your content...
Please wait while we load your content...