Selective dihydroxylation of methyl oleate to methyl-9,10-dihydroxystearate in the presence of a recyclable tungsten based catalyst and hydrogen peroxide
Abstract
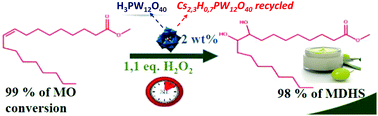
The dihydroxylation of fatty methyl esters is of prime importance for the synthesis of surfactants and lubricants. The conversion of methyl oleate (MO) to 98% yield of methyl-9,10-dihydroxystearate (MDHS) was performed in the presence of H2O2 and H3PW12O40 catalyst in the absence of a phase transfer agent. The study of the effect of hydrogen peroxide concentration revealed that significant catalytic activity was only achieved for an optimal amount of H2O2. A mechanism for this reaction was proposed where hydrogen peroxide reacts with H3PW12O40 to produce peroxo-phosphotungstate anions, which directly dihydroxylate MO. The recyclability of the catalyst was also studied. To this aim, a recyclable form of the heteropolyacid was synthesized using Cs cations (Cs2.3H0.7PW12O40). This catalyst was recycled up to three cycles without significant loss in catalytic performances.


 Please wait while we load your content...
Please wait while we load your content...