The synthesis and catalytic activity of bimetallic CuAg nanoparticles and their magnetic hybrid composite materials†
Abstract
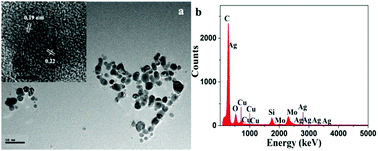
Bimetallic CuAg nanoparticles were synthesized using uric acid as a stabilizer, Fe3O4 loaded CuAg nanoparticles (Fe3O4–CuAg NPs) were also fabricated, and the morphology and content of both CuAg NPs and Fe3O4–CuAg NPs were investigated. The bimetallic CuAg NPs can be used as a catalyst and exhibited superior catalytic performance in the hydrogenation of nitrophenols and potassium ferricyanide(III). Notably, the bimetallic CuAg NPs and Fe3O4–CuAg NPs showed better catalytic activity compared with the same amount of Ag NPs in the hydrogenation of 4-nitrophenol, with rate constants k of 20.02 × 10−3 s−1 and 20.19 × 10−3 s−1, respectively. The magnetic Fe3O4–CuAg NPs can be re-used as a catalyst at least five times, promoting the concept of recycling by improving the deficiency in the disposable use of colloidal nanoparticles.


 Please wait while we load your content...
Please wait while we load your content...