Triphenylphosphonium conjugated quaternary ammonium based gel: synthesis and potential application in the efficient removal of toxic acid orange 7 dye from aqueous solution†
Abstract
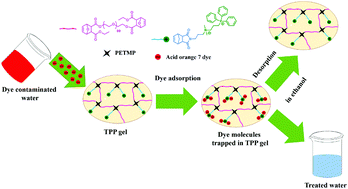
Adsorptive removal of azo dyes from textile effluents is considered to be one of the most widely used means of wastewater decontamination owing to its inexpensiveness, regeneration feasibility, and overall robustness. Most importantly, this process inherently eliminates the formation of offensive end products that eventuates further disposal problems. Therefore, it is imperative to design a suitable adsorbent that has a facile synthetic route and that features high dye removal efficiency as well as substantial regeneration. Hence, in this work, we have developed an ingenious adsorbent based on the thiol–norbornene photocrosslinking reaction. To facilitate the removal of the anionic azo dye acid orange 7, we have prudently introduced quaternary ammonium as well as triphenylphosphonium functionalities in the crosslinked network. Characterization of all the monomers and the crosslinker was accomplished by using standard spectroscopic and analytical techniques. The porous morphology of the crosslinked gel was visualized via FE-SEM and TEM analysis. Additionally, rheological studies revealed the presence of viscoelasticity in the network. Besides, the solvent uptake ability of the gel was also explored in various solvents, and it was reasonably correlated with the solubility parameter. In this regard, swelling in water was found to be particularly beneficial, as a high water uptake allowed internal adsorption sites present in the gel to be fully exposed to the dye molecules, thereby ameliorating dye removal. The material showed excellent thermal stability up to 240 °C. Further, the adsorption nature of the gel correlated well with the pseudo-second order kinetic model as well as the Freundlich adsorption isotherm model. The influence of varying temperatures (303–323 K) as well as pH (2–10) on the adsorption process was also evaluated. The maximum dye removal efficiency of 105.8 mg g−1 was obtained at 303 K. Interestingly, the material was reasonably recyclable up to 10 cycles with no significant loss of re-adsorption efficiency (around 7% loss between the 1st and 10th cycle). We envisage that this strategy of developing low-cost, efficient adsorbents will expand the scope of utilizing environmentally friendly thiol–norbornene photo click chemistry to alleviate water pollution.


 Please wait while we load your content...
Please wait while we load your content...