Copper-catalyzed cascade three-component azide–alkyne cycloaddition/condensation/transesterification: easy access to 3-triazolylcoumarins†
Abstract
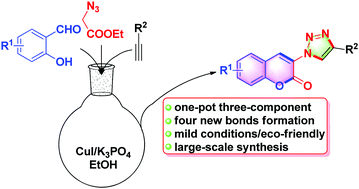
A novel and efficient strategy has been developed for the synthesis of 3-triazolylcoumarins in a one-pot, copper-catalyzed multicomponent reaction involving a cascade reaction of salicylaldehydes, ethyl 2-azidoacetate, and arylacetylenes including azide–alkyne cycloaddition, Knoevenagel condensation and an intramolecular transesterification sequence mediated by a base. This one-pot procedure provides a convenient and eco-friendly method of accessing a wide range of substituted 3-trizolylcoumarins in moderate to excellent yields with a broad substrate scope. A possible mechanism for the reaction pathway was also proposed. In addition, the fluorescence properties of 3-triazolylcoumarin derivatives were also evaluated and they displayed a wide range of fluorescence emission.


 Please wait while we load your content...
Please wait while we load your content...