Beckmann rearrangement catalysis: a review of recent advances
Abstract
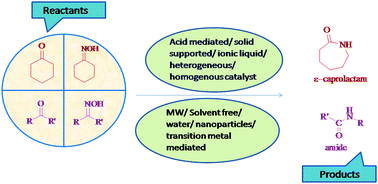
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is an acid catalyzed rearrangement of an oxime to an amide. The Beckmann rearrangement is an elegant transformation, and has been used with great success in the synthesis of natural products and pharmaceuticals. The Beckmann rearrangement has been widely used in synthetic organic chemistry; for example, the large-scale production of Nylon-6 is based on the synthesis of ε-caprolactam from cyclohexanone oxime. In this review, we will comprehensively discuss the role of different catalysts as well as different medium for the Beckmann rearrangement. The development of Beckmann rearrangement catalysis from hazardous to greener catalysts has led to tremendous improvement in their potential over the last 20 years prompting their greater application. The different catalytic systems will be revised considering both catalytic performances and synthetic aspects highlighting also their advantages and disadvantages.
- This article is part of the themed collection: 2020 Focus and Perspective articles


 Please wait while we load your content...
Please wait while we load your content...