Diaminomaleonitrile-functionalized gelators in F−/CN− sensing, phase-selective gelation, oil spill recovery and dye removal from water†
Abstract
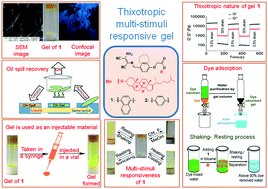
Diaminomaleonitrile-based gelators 1 and 2, which describe the linking of diaminomaleonitrile as an H-bonding site to the cholesterol unit via an aromatic linker (naphthalene/benzene), have been synthesized. They form gels from various organic and aqueous organic solvents. Aggregation of the gelators occurs due to several weak forces (e.g., hydrophobic interactions, H-bonding and π-stacking interactions) in solutions. Compound 1 is a superior gelator as compared to 2 and is capable of forming gels from 15 out of the 20 solvents tested. The gelators show multiple applications. Toluene and 1,2-dichlorobenzene gels of 1 and 2, respectively, can sense F− and CN− anions by showing gel-to-sol transformation. These ions are distinguished with the aid of Fe3+. In the designs, changes in the linker group (naphthalene/benzene) influence the gelation behaviors and gel properties (mgc, thermal stability, morphology and mechanical behavior) of the gelators. The naphthyl analogue 1 exhibits self-healing behavior in contrast to the benzene analogue 2. Both 1 and 2 show phase-selective gelation (PSG) from biphasic mixtures of water and hydrocarbons/oils. The toluene gel of 1 serves as an excellent injectable material and has been applied in environmental remediation (e.g., oil spill recovery and removal of water-soluble toxic dyes).


 Please wait while we load your content...
Please wait while we load your content...