Effect of anions on the solid-state interplay of symmetric and unsymmetric phosphonium cations†
Abstract
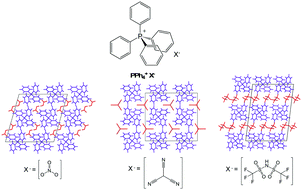
Eight organic salts made up of two different organic phosphonium monocations, tetraphenylphosphonium or benzyltriphenylphosphonium, and common anions of different shape, size and charge distribution have been structurally authenticated. The crystal packing for the tetraphenylphosphonium cations involves one-dimensional infinite stacked columns incorporating translational quadruple embraces (TQPE) regardless of the type of counter ion. As for benzyltriphenylphosphonium salts, different packing arrays were obtained with different anions. Interestingly, the anions control the intermolecular packing of the same phosphonium cation, while hydrogen bonding is found to be the dominant contributor in the overall self-assembly. Hydrophobic interactions between the phosphonium cations driven by the phenyl–phenyl embrace involving π⋯π and/or C–H⋯π interactions also contribute to the cohesion of the structures. Charge-assisted hydrogen bonding and halogen bonding were also evident, which are relatively strong compared to classical hydrogen bonds, as judged from the short intermolecular distances. The interactions have also been quantified using Hirshfeld surfaces analyses.


 Please wait while we load your content...
Please wait while we load your content...