Catalytic isomerization–hydroformylation of olefins by rhodium salicylaldimine pre-catalysts†
Abstract
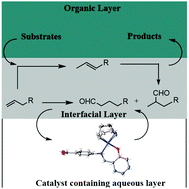
A series of new Schiff-base rhodium(I) water-soluble complexes (C1–C3), were prepared and characterized. These complexes served as catalyst precursors for the hydroformylation of 1-octene and resulted in excellent substrate conversions (>98%) with 100% chemoselectivities to aldehydes, under mild conditions. Notably, good regioselectivities towards branched aldehydes were observed clearly demonstrating the catalysts’ ability in thermodynamically favoured isomerization followed by hydroformylation (n/iso ratio ranging between 0.7–1.2). Interestingly, catalyst C1 uniquely promoted contra-thermodynamic isomerization of 2-octene to 1-octene with up to 50% conversion. The efficacy of catalyst C1 was further evaluated in the hydroformylation of longer chain olefins (C10–C12), methyl acrylate, ethyl acrylate and styrene. The catalyst displayed conversions >99% with the long chain substrates and much lower conversions with the acrylates. These water-soluble (pre)catalysts were recycled up to three times with no significant loss in catalytic activity and selectivity. Mercury poisoning tests were conducted and the experiments revealed that the conversion of the substrates into aldehydes was due to molecular active catalysts and not as a result of colloidal particles that could have formed in situ through the decomposition of the catalyst precursor. Finally, the molecular catalyst responsible for activity was established using preliminary computational calculations.


 Please wait while we load your content...
Please wait while we load your content...