Combined experimental and theoretical studies on a phenyl thiadiazole-based novel turn-on fluorescent colorimetric Schiff base chemosensor for the selective and sensitive detection of Al3+†
Abstract
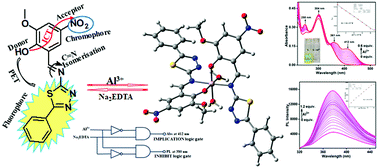
Herein, we have presented a phenyl thiadiazole-based Schiff base receptor, 2-[(Z)-(5-phenyl-1,3,4-thiadiazol-2-ylimino)methyl]-6-methoxy-4-nitrophenol (L) for the first time for turn-on fluorescent, colourimetric detection of Al3+ ions. The chemosensor L displayed very quick responses, excellent selectivity and sensitivity towards Al3+ ions in methanol–Tris–HCl buffer medium (10 mM, pH 7.2, and 1 : 1 v/v) with colourimetric and fluorometric detection limit values of 1.43 × 10−7 M and 1.15 × 10−7 M, respectively. The binding stoichiometry was obtained as 2 : 1 from the Job plot analysis and ESI-MS spectra with the association constant value of 1.76 × 102 M−1/2. The experimental results have further been supported by thorough DFT and TD-DFT studies. The chemosensor L can be applied for the formation of binary logical devices, recovery of contaminated water samples and smartphone-based chemical analysis.


 Please wait while we load your content...
Please wait while we load your content...