1H-Benzimidazole-5-carboxamidine derivatives: design, synthesis, molecular docking, DFT and antimicrobial studies†
Abstract
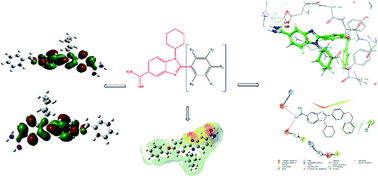
In this study, 15 new N-(cyclohexyl)-2-substituted-1H-benzimidazole-5-carboxamidine derivatives that could be new antimicrobial agents were synthesized and their antimicrobial activities were determined using the microdilution method. Some of the derivatives showed significant efficacy against MRSA and VREF with an MIC value of 8 μg mL−1 compared to reference drugs. Molecular docking studies of the compounds against PBP4 and active and allosteric regions of PBP2a were performed and estimated ADME profiles were calculated. The nitrogens of the amidine group of M7, one of the most effective antimicrobial compounds compared to reference drugs, formed two separate hydrogen bonds with ASP275 (1.77 Å) and ASP295 (1.83 Å) in the allosteric region of PBP2a. Geometric optimization parameters, MEP analysis, and HUMO and LUMO quantum parameters of M7 were calculated using DFT/B3LYP theory and the 6-311G(d,p) basis set and the results are displayed.


 Please wait while we load your content...
Please wait while we load your content...