Unusual chemistry of Cu(ii) salan complexes: synthesis, characterization and superoxide dismutase activity†
Abstract
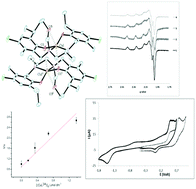
The diaminobis(phenolato) “salan” ligand precursors, N,N′-dimethyl-N,N′-bis-(2-hydroxy-3-X-5-Y-6-Z-benzyl)-1,2-diaminoethane {X = iPr, Y = Cl, Z = Me (H2L1); X = OCH3, Y = allyl, Z = H (H2L2)} and N,N′-bis-(2-hydroxy-3-X-5-Y-6-Z-benzyl)-4-methylimidazolidine {X = iPr, Y = Cl, Z = Me (H2L3); X = tBu, Y = CH3, Z = H (H2L4)} and their corresponding Cu(II) complexes are synthesized and characterized. [Cu(L1)(DMSO)] and [Cu(L2)] show the expected geometry and coordination behavior. However, upon coordination to the Cu(II) centre in the presence of CH3OH, ring cleavage in H2L3 and H2L4 occurs. Subsequent and unexpected coordination of the in situ formed pendant acetal fragment of the new ligand, formed via ring opening, leads to the formation of the phenolato bridged dimeric Cu(II) complex, [Cu(L3A)]2, and a monomeric Cu(II) complex [Cu(L4A)], where an extra acetal fragment is present as a pendant group. An outline of the probable mechanism for this process is proposed. The superoxide dismutase activity of the Cu(II) complexes is also investigated.


 Please wait while we load your content...
Please wait while we load your content...