Conjugated small organic molecules: synthesis and characterization of 4-arylpyrazole-decorated dibenzothiophenes†
Abstract
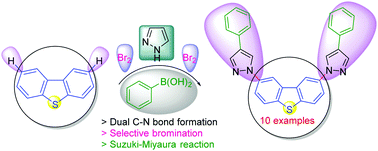
4-Arylpyrazole-decorated dibenzothiophenes have been synthesized. The synthesis involves the treatment of 2,8-dibromodibenzothiophene with pyrazole under Ullmann-type amination conditions for the dual C–N bond formation. The selective bromination of the resulting dual C–N bonded 2,8-dipyrazolyldibenzothiophene, and the subsequent Suzuki–Miyaura reaction furnished the target conjugated materials comprising small organic molecules. The electron-deficient dibenzothiophene-S,S-dioxide core was obtained in quantitative yield. UV-Vis absorption studies indicated changes in the positions of the peak maxima upon increasing the conjugation length. Electrochemical and theoretical investigations of selected synthesized materials are also included.


 Please wait while we load your content...
Please wait while we load your content...