A gram scale selective oxidation of 5-hydroxymethylfurfural to diformylfuran in the presence of oxone and catalyzed by 2-iodobenzenesulfonic acid†
Abstract
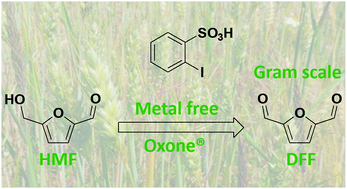
In the present work, an alternative system of 5-hydroxymethylfurfural (HMF) oxidation was studied, in an attempt to avoid the use of expensive metal catalysts, polluting systems and high pressures. In this context, the partial oxidation of the hydroxyl group on the HMF molecule leads to the formation of the corresponding aldehyde, 2,5-diformylfuran (DFF). This reaction was catalyzed by 2-iodobenzenesulfonic acid in the presence of oxone. Under optimized experimental conditions, the HMF conversion was found to be 100%, while the DFF yield and selectivity were almost 90%.


 Please wait while we load your content...
Please wait while we load your content...