Structure–property relationship studies of 3-acyl-substituted furans: the serendipitous identification and characterization of a new non-classical hydrogen bond donor moiety†
Abstract
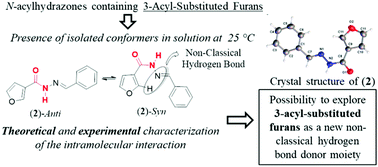
Conformational stabilization mediated by hydrogen bonds can play an important role in the drug-target recognition process. Non-classical hydrogen bonding is receiving great attention, where C–H⋯Y hydrogen bonds are found in some biological systems and are important for molecular recognition, leading to the possibility of exploration of this interaction in drug design projects. Studies that identify and characterize new non-classical interactions in different systems can assist in the rational application of these interactions. In this paper, we describe the serendipitous identification and characterization of a new non-classical hydrogen bond donor moiety found in 3-acyl-substituted furan. We applied a theoretical and experimental approach in the study of several N-acylhydrazones containing 3-acyl-substituted furan, where we describe the presence of a hydrogen bond between the C(2)–H bond of 3-acyl-substituted furan with the imine nitrogen in the syn conformer of the N-acylhydrazone moiety. In addition, we evaluated the strength of this interaction with intermolecular models. Thus, we believe that the 3-acyl-substituted furan moiety can be rationally explored as a hydrogen bond donor moiety in drug design projects.


 Please wait while we load your content...
Please wait while we load your content...