Can non-heterocyclic hydrophobic amino acids when tethered at the C-terminus of 12-hydroxy stearic acid-based amphiphilic derivatives drive hydrogelation propensity effectively?†
Abstract
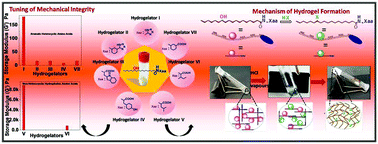
A new amphiphilic construct comprising 12 hydroxy stearic acid (12-HSA) at the N-terminus, appended to various aromatic heterocyclic scaffolds (hydrogelators I–IV) either in the backbone or sidechain, displayed excellent hydrogel forming abilities, selectively in the presence of hydrogen chlorides/bromides be it in the form of liquids or vapours (A. Sharma, A. Gupta, P. Tiwari, A. Basu and A. Dutt Konar, New J. Chem., 2020, 44, 3828). In an effort to explore the influence of hydrophobic, non-heterocyclic residues in the gelation process, herein we report the design and synthesis of hydrogelators V–VII, with the general structure 12-HSA-Xaa (Xaa: Leu (V), Ile (VI), mABA (VII)), that exhibited self-aggregation in a fashion akin to that of the reported molecules. As confirmed by DFT calculations and experimental investigation, the halides of the amphiphilic derivatives are held responsible for promoting the gelation phenomena. To confirm the selectivity towards HCl/HBr, we used numerous other organic and inorganic acids, but ended up with negative inference in every case. Interestingly, our rheological experiments revealed the mechanical integrity of hydrogelators V–VI to be significantly higher in comparison to other compounds. The conformational analysis using FT-IR spectroscopy & powder X-ray diffraction studies allowed us to conclude the hydrogelators to be organized in a β-sheet like orientation. Additionally, morphological analysis using FE-SEM, highlighted that for hydrogelators V & VI, the participation of hydrophobic and van der Waals interactions as the driving force, might have led to the lateral association of filamentous fibrils, unlike others (heterocyclic derivatives; hydrogelators I–IV), thereby contributing to the increased mechanical strength. In the era of removal of toxic effluents, we speculate that the discovery of this novel class of amphiphilic derivatives holds promise for the future cleanup of hydrogen halides from the atmosphere, thus providing a green strategy for effluent management.


 Please wait while we load your content...
Please wait while we load your content...