A photochemical transformation of cyclic peptides leading to formation of selenolanthionine bridges†
Abstract
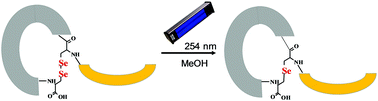
Lanthionine is an important, non-proteinogenic amino acid occurring among others in peptide antibiotics–lantibiotics. The majority of known and time-consuming synthetic methods require the application of non-standard amino acid derivatives. Here, we report a convenient, straightforward and efficient photochemical conversion of selenocysteine-based diselenide bridge to selenolanthionine – reaction driven by UV light.


 Please wait while we load your content...
Please wait while we load your content...