Syntheses, structures and magnetic properties of novel tetrameric Ln2Mn2 and ring-like Ln4Mn4 clusters†
Abstract
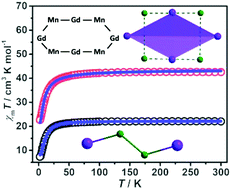
Three tetrameric Ln–Mn clusters with formulae [Ln2Mn2(hmp)6(NO3)4(H2O)4](ClO4)2·xH2O (Ln = Y, x = 1 (1); Ln = Gd, x = 3 (2); Ln = Dy, x = 4 (3)) and three ring-like Ln–Mn clusters [Ln4Mn4(hmp)4(L)4(H2L)4(CH3CN)(H2O)7](NO3)4·H2O (Ln = Y (4), Gd (5), Dy (6), hmpH = 2-(hydroxymethyl)pyridine, H3L = 2,2-bis(hydroxymethyl)butyric acid) have been synthesized in the presence of alcohol ligands. Single X-ray analysis revealed that compounds 1–3 exhibited a zigzag metal core unit of [Y2Mn2(hmp)6]6+ ([Y–Mn–Mn–Y]), which could form a three-dimensional (3D) metal framework via π–π stacking. Compounds 4–6 formed the ring-shaped metal skeleton of Ln4Mn4 and displayed an ideal metal arrangement with alternating Ln and Mn ions, which was consistent with the predicted metal arrangement of [–Ln–M–Ln–M–] with weak magnetic interactions. Investigation into the magnetic properties showed that 1 displayed significant ferromagnetic interactions and 2–6 displayed dominant antiferromagnetic interactions. Magnetic calculations showed that in 2, Gd⋯Mn and Mn⋯Mn presented an antiferromagnetic interaction and a weak ferromagnetic interaction, respectively. For 5, Gd⋯Mn showed a weak antiferromagnetic interaction and Gd⋯Gd showed a much weaker ferromagnetic interaction due to the spacing of Gd(III) ions by Mn(III) ions, which effectively weakened the antiferromagnetic coupling between Gd3+ ions. In addition, magnetothermal studies showed that 5 displayed magnetic entropy changes of 15.5 J kg−1 K−1 at 3.5 K for ΔH = 7 T.


 Please wait while we load your content...
Please wait while we load your content...