Magnetic and thermal studies of a coordination polymer: bis(glycolato)nickel(ii)†
Abstract
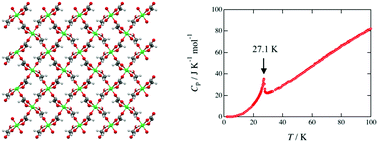
A coordination polymer, bis(glycolato)nickel(II) ([Ni(HOCH2COO)2]), has an S = 1 Heisenberg two-dimensional square planar-lattice type magnetic network. Single crystals of this material were successively grown from aqueous solutions including nickel acetate and glycolic acid in air. Low-temperature structural analyses revealed that this material showed no structural phase transition in the low temperature region, which was observed in the copper derivative, isostructural with this material. Magnetic measurements revealed the dominance of antiferromagnetic interactions with J/kB = 15.8 K and an anomaly at approximately 30 K that correspond to a paramagnetic-to-antiferromagnetic ordering transition. Heat-capacity measurements using the single crystals found a λ-type anomaly at 27.1 K, associated with the magnetic transition. The magnetic entropy ΔS was 8.85 J K−1 mol−1, which is close to the theoretical value for the magnetic ordering of S = 1 spin, 9.13 J K−1 mol−1. Magnetic properties of [M(HOCH2COO)2] (M = Mn(II), Co(II), Ni(II), and Cu(II)) drastically depend on their metal ions.


 Please wait while we load your content...
Please wait while we load your content...