Ruthenocycles of benzothiazolyl and pyridyl hydrazones with ancillary PAHs: synthesis, structure, electrochemistry and antimicrobial activity†
Abstract
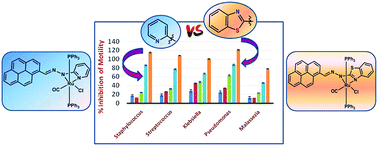
Two types of bivalent ruthenium complexes [RuLPy(CO)Cl(PPh3)3] 3 and [RuLBenz(CO)Cl(PPh3)3] 4 were synthesized starting from [RuH(CO)Cl(PPh3)3] and heterocyclic hydrazone ligands 1 and 2 respectively. X-ray diffraction studies reveal that in both types of complexes, the ligands behave as monoanionic bidentate Nhydrazonyl and Npyridyl/Nbenzothiazolyl donors towards ruthenium(II), thereby furnishing four-membered metallacycles. The multiple transitions in the electronic spectra have been elucidated by time dependent density functional theory (TDDFT). The redox active nature of both 3 and 4 has been substantiated from the well-defined oxidative responses and theoretical scrutiny corroborates that one of them is exclusively ligand centred while the other one is chiefly due to the RuII/RuIII oxidation. Both the types of complexes exhibit a significant antimicrobial activity, although the activity of 4 is more prominent, particularly over Pseudomonas. These are analyzed by measuring ZOI, MIC as well as the extent of membrane damage and protein leakage studies. The complexes probably cause free-radical facilitated oxidative damage to the bacterial cells during the course of their activity.


 Please wait while we load your content...
Please wait while we load your content...