The effect of vicinal di-halo substituents on the organogelling properties of aromatic supramolecular gelators and their application as soft templates†
Abstract
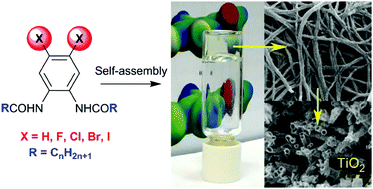
A pronounced effect of vicinal dihalogen substituents on the gelling properties of aromatic low molecular weight organogelators is reported. A new family of N,N′-(4,5-dihalogen-1,2-phenylene)dialkylamides with fluorine, chlorine, bromine and iodine was designed and synthesized. A systematic investigation of their organogelling ability, thermic stability, mechanical properties and self-assembled structure was performed to elucidate the effect that the vicinal di-halo substituents have on the organogels. It was found that the presence of two halogen atoms (X) has a determinant effect as the brominated compounds are generally the most efficient organogelators. In hydrocarbons, the gelling ability increased from fluorine to iodine following the halogen bond donor ability trend. SAXS results were in agreement with a fibrillar self-assembly where the halogens are located at the surface of the fibers. Multiple cooperative interactions are involved in the self-assembly of the gels: π–π stacking, hydrogen bonds and X⋯X contacts. Thus, this work provides a new strategy for the design of new gelators or to improve the efficiency of known organogelators by introducing two vicinal halogen substituents into the aromatic rings. An ethanolic gel was also successfully used as a template to prepare silica and titania nanotubes. Hence, such organogels are promising materials for future research and development.


 Please wait while we load your content...
Please wait while we load your content...