Antioxidant properties of ethenyl indole: DPPH assay and TDDFT studies†
Abstract
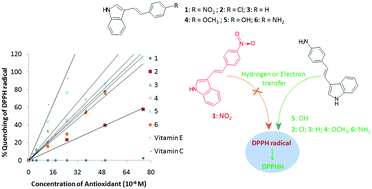
A series of ethenyl indoles (e.g. 3-(4-substituted phenylethenyl-E)-N–H-indole) with various donor or acceptor substituents have been synthesized and their antioxidant properties have been studied. Ethenyl indoles exhibit antioxidant activity in a substituent dependent manner. Ethenyls bearing strong electron withdrawing substituents show weak or no antioxidant activity, whereas ethenyls with electron donating substituents exhibit antioxidant properties comparable to vitamin E. It can be seen from a plot of the percentage of inhibition versus the antioxidant concentration, that the hydroxy substituted ethenyl indole exhibits good antioxidant properties (50% inhibition concentration (IC50) ∼ 24 μM) as compared to the other ethenyls (IC50: 30–63 μM) and that it is comparable to vitamin E (IC50 ∼ 26 μM). The results are also supported by the computational data obtained through time dependent density functional theory (TDDFT) calculations. From the TDDFT and antioxidant study, it was shown that there is a correlation between the highest occupied molecular orbital (HOMO)–lowest unoccupied molecular orbital (LUMO) energy, the ground state dipole moment, optical band gap, bond dissociation enthalpy and the ionization potential of the ethenyls with the antioxidant properties. A possible hydrogen and/or electron and proton transfer mechanism is suggested for the quenching of the free radical.


 Please wait while we load your content...
Please wait while we load your content...