First principles study of SiC as the anode in sodium ion batteries
Abstract
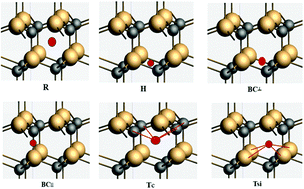
The application of sodium ion batteries (NIB) for use as rechargeable energy storage devices is being researched due to limited knowledge on electrode materials. The energy requirements for electrical appliances and issues related to contemporary ion batteries motivated us to search for potential materials as anodes in NIBs. This work is carried out using density functional theory implemented in ADF-BAND code with the motivation to explore the potential of silicon carbide (SiC) as an anode material to be used in NIBs. Detailed investigations were performed to explore the structural, electronic and transport properties of pure, vacancy containing and Na intercalated 3C-, 4H- and 2H-polytypes of SiC. The energy profiling revealed that the R-interstitial site is the most favorable site for 2H and 4H, whereas the Tc-interstitial is a suitable site for 3C polytypes. The pure SiC is found to be unfavorable for Na intercalation, whereas mono- and di-vacancies of silicon appeared to provide a plausible strategy to meet the objectives. The diffusion study performed through the nudged elastic band method revealed that the migration path along the z-direction offers a minimum barrier energy for sodium migration compared to other paths. The values of the intercalation voltage and storage capacity pointed towards the aptness of hexagonal SiC for use as an anode material in NIBs.


 Please wait while we load your content...
Please wait while we load your content...