Iodine-catalyzed α,β-dehydrogenation of ketones and aldehydes generating conjugated enones and enals†
Abstract
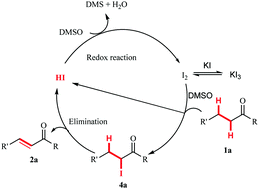
A transition metal-free α,β-dehydrogenation of ketones and aldehydes was developed. This reaction was conducted in a facile I2/KI/DMSO system to produce the corresponding unsaturated compounds in good to high yields. The gram-scale experiment also indicated the potential synthetic value of this new reaction in organic synthesis. In the reaction, DMSO acted as both solvent and mild oxidant.


 Please wait while we load your content...
Please wait while we load your content...