Pyrenoimidazole-fused phenanthridine derivatives with intense red excimer fluorescence in the solid state†
Abstract
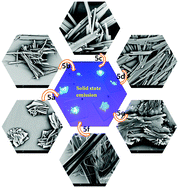
A new series of polycyclic aromatic hydrocarbons (PAHs), namely, various pyrene-fused phenanthridines PyFPs having different substituents were developed as fluorescent emitters for optoelectronic applications. The compounds were synthesized via transition metal-free, base-promoted intramolecular C–C bond formation between pyrene and phenanthridine bridged by imidazole, which facilitated extended π-conjugation throughout the molecule. PyFPs also maintained the vibronic feature characteristic of PAHs with intense blue emission in the solution state, with the spectral maximum at around 425 nm. The absorption spectra of the drop-cast film of PyFPs maintained marginal spectral shifts with respect to that of the solution; however, the fluorescence spectra were quite phenomenal, with the 25 to 107 nm red-shifted spectral maximum showing intense red emission. Fluorescence, electron, and atomic force microscopy images revealed the formation of rod- and flower-like self-assembled supramolecular structures possibly aided by strong intermolecular π–π interactions. Solid-state fluorescence was also observed in the red region with the broad spectra spread over the near-infrared region. The largely red-shifted emission maximum was attributed to the excimer emission in the solid state formed through tailored intermolecular interactions by pyrene and phenanthridine. These have the potential to be used in optoelectronic applications.


 Please wait while we load your content...
Please wait while we load your content...