Construction of a family of Ln3 clusters using multidentate Schiff base and β-diketonate ligands: fluorescence properties, magnetocaloric effect and slow magnetic relaxation†
Abstract
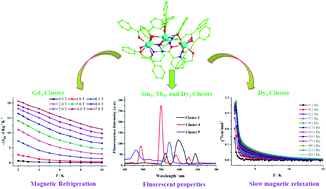
A family of μ-Oacylhydrazone-bridged Ln3 clusters, [Ln3(dbm)5(HL)2]·x[solvent] (Ln = Nd (1), Sm (2), Gd (3), Tb (4), Dy (5), Ho (6), and Er (7), H3L = N′-(2-hydroxybenzylidene)-6-(hydroxymethyl)picolinohydrazide, and Hdbm = dibenzoylmethane), was successfully obtained by using a multidentate hydrazone ligand and lanthanide β-diketone salts via a solvothermal method. The seven Ln3 clusters were characterized by performing infrared spectroscopy (IR), elemental analysis (EA), thermogravimetric analysis (TGA), UV-Vis spectroscopy and single-crystal X-ray diffraction. Structural analyses showed clusters 1–7 to be isostructural and to mainly contain three Ln(III) ions connected through four μ-Ohydrazone bridges. The magnetism investigations indicated showed cluster 3 displaying cryogenic magnetic refrigeration with a −ΔSm of 20.60 J K−1 kg−1 (T = 2.0 K, ΔH = 7.0 T); while cluster 5 showed slow magnetic relaxation with a ΔE/kB of 20.47 K and τ0 of 1.4 × 10−8 s. Moreover, solid-state fluorescence analyses showed clusters 2, 4 and 5 displaying the characteristic lanthanum luminescence at room temperature.


 Please wait while we load your content...
Please wait while we load your content...