Non-photoisomerizable butterfly shaped tetrasubstituted azobenzenes: synthesis and photophysical studies†‡
Abstract
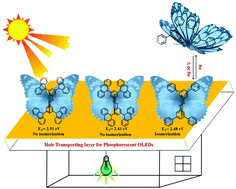
The trans-azobenzene (AB) substituted derivatives with bulky carbazole (1), phenothiazine (2), and phenyl (3) have been synthesized to understand the effect of steric crowding around the –N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– linkage on the photoisomerization process. The substituents maintain orthogonality to the azobenzene plane and the electronic properties exhibit a combination of individual chromophores. Irradiation of 1 and 2 with various light sources including a 365 nm pen-ray lamp, and a 150 W Xe–Hg lamp, and even exposure to sunlight for a week does not yield the cis isomer revealing the exceptional photostability of the synthesized azobenzene derivatives. These studies suggest that possessing steric crowding around the azo linkage would be a potential strategy to develop photostable azobenzene-based functional materials.
N– linkage on the photoisomerization process. The substituents maintain orthogonality to the azobenzene plane and the electronic properties exhibit a combination of individual chromophores. Irradiation of 1 and 2 with various light sources including a 365 nm pen-ray lamp, and a 150 W Xe–Hg lamp, and even exposure to sunlight for a week does not yield the cis isomer revealing the exceptional photostability of the synthesized azobenzene derivatives. These studies suggest that possessing steric crowding around the azo linkage would be a potential strategy to develop photostable azobenzene-based functional materials.


 Please wait while we load your content...
Please wait while we load your content...