Thiophene backbone-based polymers with electron-withdrawing pendant groups for application in organic thin-film transistors†
Abstract
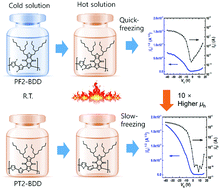
Two thiophene backbone-based hole-transporting polymers, namely, poly[(2,2′-bithiophene-5,5′-diyl)-alt-(5,7-bis(2-butyloctyl)-4H,8H-benzo[1,2-c:4,5-c′]dithiophene-4,8-dione-1,3-diyl)] (PT2-BDD) and poly[(3,3′-difluoro-[2,2′-bithiophene]-5,5′-diyl)-alt-(5,7-bis(2-butyloctyl)-4H,8H-benzo[1,2-c:4,5-c′]dithiophene-4,8-dione-1,3-diyl)] (PF2-BDD), were prepared by using electron-withdrawing pendant groups such as ketone and fluorine moieties. They both exhibited a planar backbone with efficient π conjugation, which is suitable for hole transport in organic thin-film transistors (OTFTs). However, the fluorinated one (i.e., PF2-BDD), despite its perfect backbone planarity and strong intra- and intermolecular interactions, could not enhance the OTFT performance; due to its solvent resistance, electron negativity, and random orientation, PF2-BDD showed 10 times lower hole mobility than the non-fluorinated polymer (i.e., PT2-BDD). Nonetheless, the two-dimensional grazing incidence X-ray diffraction and temperature-dependent absorption spectra of the synthesized polymers provided crucial information to understand the relationship between their hole transport behavior and molecular structure.


 Please wait while we load your content...
Please wait while we load your content...