A mechanistic study of the activation of small molecules (H2 and C2H2) by group 14 analogues of selenophene†
Abstract
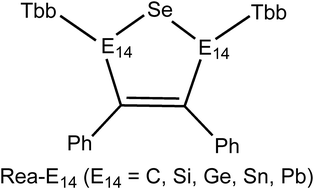
In this study, the reactivity influenced by group 14 elements (E = C, Si, Ge, Sn, and Pb), which are used as substituents in heterocyclic five-membered rings, was theoretically examined by using density functional theory (B3PW91/def2-SVP). For this purpose, five group 14 heterocycles containing a selenium atom, Rea-E, have been chosen as model reactants in this work. Theoretical evidence revealed that the electronic effects of group 14 elements played a crucial role in forming new optoelectronic materials with a heteroatomic five-membered ring, which could be an interesting synthetic target for the next generation. Moreover, the addition reactions for Rea-E with dihydrogen as well as acetylene along with the reaction coordinates were quantitatively analyzed by using the EDA–NOCV (energy decomposition analysis–natural orbitals for chemical valence) method in this study. Theoretical examinations demonstrated that both the activation barrier and reaction energy were strongly dependent on the atomic radius of the E atom due to the Pauli repulsion. Since the carbon element possessed the smallest atomic radius in the group 14 family, the addition reactions of Rea-C with H2 as well as acetylene can lead to the largest activation barrier and an endothermic reaction.


 Please wait while we load your content...
Please wait while we load your content...