Experimental and computational studies on the formation and biological properties of the simplest polyfluoroalkyl phosphonates†‡
Abstract
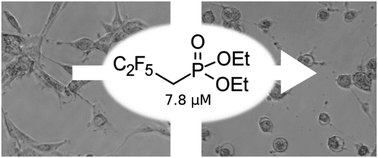
A representative set of the simplest dialkyl polyfluoroalkylphosphonates was in silico profiled against their absorption, distribution, metabolism, and excretion with the use of the SwissADME tool. Promising results of the screening led us to attempt to synthesize the title compounds for further biological investigations. Detailed experimental and quantum-theoretical (DFT) investigations were performed to reveal that the studied compounds could not be obtained through the standard Michaelis–Arbuzov or Michaelis–Becker reactions. Kinetic studies showed that trimethyl phosphite undergoes a reaction with 1,1,1-trifluoro-2-iodoethane several orders of magnitude slower than a side reaction. The difficulty was overcome by developing a simple three-step synthesis path that involves only readily available substrates. The newly synthesized substances were tested against several cell lines. The in vitro research revealed that both dimethyl and diethyl (2,2,3,3,3-pentafluoropropyl)phosphonate exhibited toxicity towards glioblastoma cells (U-87 MG) at a considerably lower concentration than known chemotherapeutics.


 Please wait while we load your content...
Please wait while we load your content...