A donor design strategy for triazine-carbazole blue thermally activated delayed fluorescence materials†
Abstract
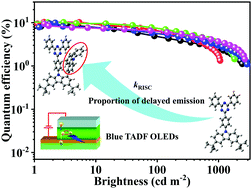
The largely twisted intramolecular charge transfer (TICT) framework is the most commonly adopted strategy for constructing thermally activated delayed fluorescence (TADF) molecules. By increasing the dihedral angle between the donor and acceptor, a small singlet–triplet energy splitting (ΔEST), high reverse intersystem crossing rate (kRISC) and large proportion of delayed emission can be achieved simultaneously. Five twisted molecules, tCPT, Ph-tCPT, o-PhCz-tCPT, p-PhCz-tCPT and 3-PhCz-tCPT, are designed and synthesized combining cyaphenine and 3,6-di-tert-butyl-9H-carbazole or its derivatives. Phenyl (Ph) and N-phenyl carbazole (N-PhCz) are introduced to the 1-site of 3,6-di-tert-butyl-9H-carbazole to design the donor units, with the expectation to tune the dihedral angle between the donor and acceptor and the frontier molecular orbital distribution. As a result, the ΔEST decreases from 0.30 eV for tCPT to 0.20 eV for 3-PhCz-tCPT, and the delayed fluorescence (DF) proportion increases from 19.5% for tCPT to 31.9% and 37.3% for o-PhCz-tCPT and 3-PhCz-tCPT. Organic light-emitting diodes (OLEDs) using these molecules as doped emitters exhibit pure blue emission with the peaks at 456–466 nm. The emitters with higher DF proportions show higher electroluminescence efficiencies, e.g. 13.8 and 16.4 cd A−1 for o-PhCz-tCPT and 3-PhCz-tCPT devices, respectively, representing an efficiency increase by 10% relative to tCPT. It is demonstrated that incorporation of bulky substituents at the 1-site of carbazole is an effective and practical way to facilitate RISC and enhance the DF proportion of blue TADF materials.


 Please wait while we load your content...
Please wait while we load your content...