One step conversion of 1,5-bis(dimethylamino)naphthalene to salts of “back to back” bis-acridine derivatives†
Abstract
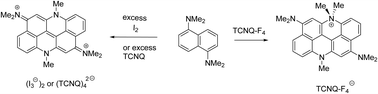
Oxidation of 1,5-bis(dimethylamino)naphthalene with iodine leads directly to a bis(dimethyliminium) derivative of acridino[2,1,9,8-klmna]acridine, containing six fused six-membered rings, as a bis triiodide salt. The cation has a twisted structure due to the minimisation of peri interactions between each dimethyliminium group and a hydrogen atom. Use of TCNQ as oxidizing agent leads to the same dication as a tetrakis(TCNQ) salt, while use of TCNQ-F4 gave a related monocation which is dimethylated on a ring nitrogen atom.


 Please wait while we load your content...
Please wait while we load your content...