B-site cobalt-doped perovskite oxide BaNiO3 oxygen sorbents for performance improvement of oxygen enriched gas production
Abstract
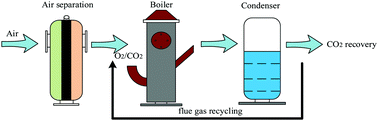
B-site cobalt-doped perovskite oxide BaNiO3 oxygen sorbents have been synthesized by an EDTA sol–gel method and further applied for producing oxygen. BaCoxNi1−xO3−δ oxygen sorbents were characterized by scanning electron microscopy (SEM) and cyclic oxygen adsorption/desorption experiments. Results showed that BaCo0.6Ni0.4O3−δ features higher production amounts of oxygen among the candidates. The operating factors of oxygen releasing performance were investigated in detail including adsorption temperature, desorption temperature, adsorption time, CO2 flow rate and CO2 partial pressure. It was concluded that the temperature and CO2 flow rate are the main reasons affecting the performance of perovskite oxygen sorbents. The optimal operating parameters of oxygen releasing performance for BaCo0.6Ni0.4O3−δ were as follows: adsorption temperature was 850 °C, desorption temperature was 850 °C, air adsorption time was 30 min, CO2 flow was 200 ml min−1 and the ratio of CO2/N2 was 100 : 0. The oxygen production amount of BaCo0.6Ni0.4O3−δ was 49.5 mg g−1 under the optimal reaction conditions. Finally, multiple cycles demonstrated that BaCo0.6Ni0.4O3−δ displays higher stability and regeneration capacity, which is the key factor providing a stable O2/CO2 gas stream for oxyfuel combustion application.


 Please wait while we load your content...
Please wait while we load your content...