A new Mumm-type rearrangement with dithiocarbamates via isocyanide-based multicomponent reaction under ultrasound irradiation: synthesis of polysubstituted pyrrolidine compounds†
Abstract
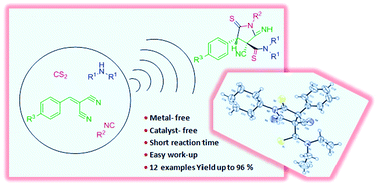
A novel and efficient multicomponent reaction for the synthesis of polysubstituted pyrrolidine derivatives is described under catalyst-free conditions using ultrasonic irradiation. The reactions were performed via a one-pot four-component condensation of secondary amines, carbon disulfide, isocyanides, and gem-dicyano olefins at room temperature to afford polysubstituted pyrrolidines diastereoselectively in 56–96% yields. This is the first report of a Mumm-type rearrangement with dithiocarbamates followed by intramolecular cyclization, which leads to the preparation of the key structure of pyrrolidine.


 Please wait while we load your content...
Please wait while we load your content...