Density functional theory investigations on the coordination of Pa(v) with N,N-dialkylamide†
Abstract
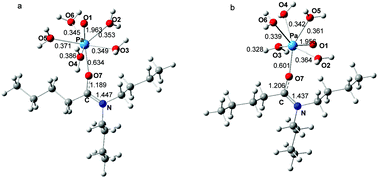
The density functional theory (DFT) method was used to study the coordination of a series of N,N-dialkylamides with Pa(V) to shed light on the inherent principles for screening amide extractants of Pa(V) from aqueous solution. The thermodynamic analysis indicates that linear alkyl substituents (on either amido or carbonyl groups) are superior over branched alkyl groups, and the introduction of alkyl substituent(s) on γ, β, and α-positions on the amido groups weakens the binding of N,N-dialkylamides with Pa(V). On this basis, possible structural design strategies for efficient extractants of Pa(V) were proposed, and the bonding characteristics between the Pa(V) center and amide were deduced.


 Please wait while we load your content...
Please wait while we load your content...