Bonding–antibonding state transition induces multiple electron modulations toward oxygen reduction reaction electrocatalysis†
Abstract
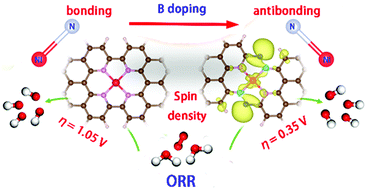
Exploring efficient electrocatalysts for the oxygen reduction reaction (ORR) is key to realize the application of fuel cells and metal–air batteries. Identifying the key intermediate and optimizing its adsorption have been a valued approach to guide the catalyst development. Here, based on the linear scaling relation between the adsorption free energy of intermediates and the finding that the antibonding state promotes the adsorption of intermediates, we rationally propose a design concept of ORR electrocatalysts: the electron donating element is applied to regulate the bonding–antibonding property, promote the electron transfer from the active site to adsorbates, optimize the adsorption of intermediates, and thus enhance the ORR activity. The calculation results indiate that B-doped Ni–N4 systems (Ni–N4–B) possess a stronger antibonding state than the Ni–N4 structure, exhibiting superior ORR activity. In particular, the Ni–N4–2B-2 nanoribbon shows a lower theoretical overpotential (0.35 V). The two-fold coordinated B doping can induce the transformation from the bonding state to the antibonding state of the Ni–N bond accompanied by the optimization of multiple descriptors towards the ORR including a higher charge and spin density, a larger d-band center value, a narrower d-bandwidth and a weaker coordination strength of Ni–N, thus greatly enhancing the electrocatalytic activity.


 Please wait while we load your content...
Please wait while we load your content...