Microwave assisted synthesis of β-keto thioethers and furan derivatives by thiol directed multicomponent reactions†
Abstract
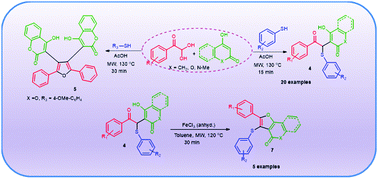
A series of β-keto thioethers tethered with cyclic-1,3-dicarbonyls (4) has been prepared by a rapid three-component reaction of arylglyoxal, cyclic 1,3-dicarbonlys and thiols in acetic acid medium under microwave heating conditions. Interestingly, under similar reaction conditions, the reaction of phenylglyoxal hydrate, 4-hydroxycoumarin and some thiols such as 4-methoxythiophenol, 2-mercaptobenzothiazole and 2-hydroxythiophenol provides the butterfly like tetra-substituted furan (5) instead of the corresponding β-keto thioethers 4. We also have developed an FeCl3 catalyzed cyclization reaction of β-keto thioethers (4) for the synthesis of fused furans (7) under microwave heating conditions. The present work demonstrates how the substituents of thiols and arylglyoxals influence the results of the three-component reaction of arylglyoxal, thiol and cyclic 1,3-dicarbonyls under acidic conditions.


 Please wait while we load your content...
Please wait while we load your content...