A sulfated/chlorinated Sr–Fe composite oxide as a novel solid and reusable superacid catalyst for oleic acid esterification†
Abstract
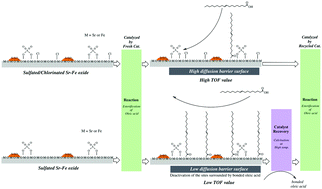
Stability and reusability are the major issues for a solid acid to be considered as an alternative for conventional homogeneous acids currently applied in industries. In this study, a novel solid superacid was prepared by implanting an alkaline earth metal, Sr, in a ferric oxide matrix. Sulfate groups were then grafted onto the prepared Sr–Fe composite oxide in order to facilitate the charge separation effect between the surface Sr cation and the adjacent oxygen anion, while Cl− ions were covalently coordinated onto the catalyst in order to suppress the inactivation caused by saponification of surface Sr cations on the catalyst when the catalyst was applied to carry out the model reaction, the esterification of oleic acid with methanol. After surface modification, the acidic properties of the prepared catalyst were characterized by TGA, pyridine FT-IR and n-butylamine back-titration. The FT-IR spectrum of the catalyst with adsorbed pyridine revealed that the surface active sites were dominated by Brønsted acid sites. The catalytic properties and XRD profiles of the surface modified Sr–Fe composite oxides provided new insights into the role of the crystalline SrSO4 cluster (which was formed after the Sr–Fe composite oxide was modified with H2SO4 and HCl) in the enhancement of catalyst reusability and reactivity. The Koros–Nowak test for the sulfated/chlorinated Sr–Fe composite oxide revealed that the reaction rate of catalytic esterification was controlled by mass transfer of the reagents over the catalyst rather than the chemical reaction, indicating the high catalytic reactivity of the active sites present on the sulfated/chlorinated Sr–Fe composite oxide. The stability and reusability testing results indicated that the sulfated/chlorinated Sr–Fe composite oxide could be recycled 20 times without considerable reactivity loss.


 Please wait while we load your content...
Please wait while we load your content...