Driving proton transfer reactions in the 2-methylfuran ring with external forces
Abstract
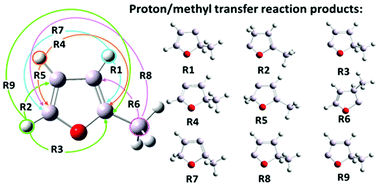
In this paper we investigate the proton transfer reactions in 2-methylfuran. This molecule has gained much attention recently as a potential candidate for biofuels and therefore its pyrolysis has been extensively studied. It is agreed that this process begins with a formation of carbenes (singlet) via the proton transfer reactions in 2-methylfuran. In this study, the proton transfer reactions are induced by specific external forces applied to the shifted protons or the carbon atoms from the methyl group. These external forces are generated using our recently proposed standard and enforced geometry optimization (SEGO) method. This approach allowed us to find automatically the final proton transfer products and to locate all relevant transition states.


 Please wait while we load your content...
Please wait while we load your content...