Amphiphilic fluorescent carbon nanodots as a selective nanoprobe for nitrite and tetracycline both in aqueous and organic solutions†
Abstract
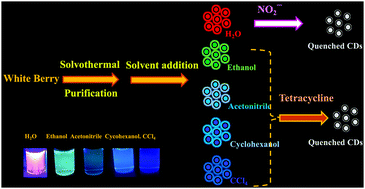
The dispersibility of carbon dots in organic and/or aqueous solvents plays a critical role in various application fields. Amphiphilic carbon dots could find broader applications due to their hydrophilic and lipophilic properties. Here, a method is described for the preparation of amphiphilic carbon dots (CDs) from white berries as a carbon source. The CDs can be well dispersed both in organic and aqueous solvents. Fluorescence is strongly red-shifted upon going from an organic solvent (in CCl4 the emission is blue with a maximum at 450 nm) to an aqueous solvent (orange fluorescence with a maximum near 600 nm). Fluorescence is independent of the excitation wavelength which is uncommon in the carbon quantum dot family. Nitrite is found to quench fluorescence in water solution, but not other common metal cations and anions. In chloroform, the fluorescence is quenched by tetracycline. The linear part of the calibration plot for nitrite covers the 5 to 90 nM concentration range, with a 1.5 nM detection limit. The linear range for tetracycline extends from 10 to 150 mM.


 Please wait while we load your content...
Please wait while we load your content...