Regioisomeric monopyridine-functionalized triarylethene: small AIEgens with isomeric effect and an efficient platform for the selective and sensitive detection of Pd2+ and Fe3+†
Abstract
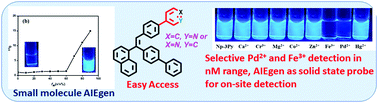
Previously, pyridine-functionalized tetraarylethenes have been established as potential AIEgens (aggregation-induced emission-active fluorogens) for numerous applications, whereas monopyridyl-linked triarylethene has been reported as a non-AIEgen. In this study, we afforded AIE-active monopyridine-functionalized unsymmetrically substituted triarylethene in which naphthyl, biphenyl, and 4-phenylpyridine rotors were attached to an alkene stator. Moreover, two regioisomeric pyridyl compounds were synthesized to study the isomeric effect on AIE properties. Both regioisomeric compounds were found to be AIE-active with slight variation. The reasons behind the AIE properties of these compounds were substantiated by scanning electron microscopy studies, lifetime measurements, and molecular packing studies in a single crystal. The relatively large number of non-covalent interactions in the 4-pyridyl isomer were slightly unfavorable for the emission of fluorescence in the aggregate state; however, these interactions were beneficial for the emission of strong fluorescence in the solid state. Both compounds were individually utilized for the naked eye detection and identification of Fe3+ and Pd2+ based on the extent of fluorescence quenching in the solution state (∼10−9 M detection limit) and the solid state (10−3 M for Pd2+ and 10−2 M for Fe3+). The quenching mechanism was found to involve the static and dynamic complexation of pyridyl N-atom with the metal ion. This fact was further proved by 1H NMR titration.


 Please wait while we load your content...
Please wait while we load your content...