Synthesis and characterization of nitrogen-based ionic liquids bearing allyl groups and examples of their application†
Abstract
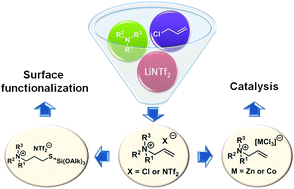
The syntheses and full spectral (NMR, MS, and IR), thermal (DSC) and ion chromatographic characterization of two series of amine-derived ionic liquids bearing allyl substituents and having chloride or bis(trifluoromethanesulfonyl)amide anions have been presented. The analysis of the dependence of the 1H NMR chemical shift values of the selected protons on the cation and anion structure has been conducted. DSC analysis records together with ion chromatography analysis results have been presented and interpreted. Moreover, a selection of obtained chloride ionic liquids have been transformed into the corresponding chlorocobaltates and chlorozincates or used as substrates in thiol–ene click reactions to indicate their potential as precursors of catalysts or various surface modifiers.


 Please wait while we load your content...
Please wait while we load your content...