Monoalkylation of aniline with trichloroacetimidate catalyzed by (±)-camphorsulfonic acid through an SN1 reaction based on dual hydrogen-bonding activation modes†
Abstract
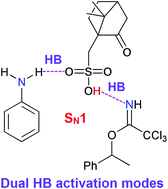
Monoalkylation of aniline can be readily achieved using trichloroacetimidate as an alkylating agent and a Brønsted acid, such as (±)-camphorsulfonic acid, as a catalyst. In this study, systematic theoretical calculations were performed to understand the reaction mechanism for the monoalkylation of 2,5-dichloroaniline with trichloroacetimidate catalyzed by (±)-camphorsulfonic acid; moreover, the judgement about whether the reaction takes place via an SN1 or SN2 mechanism was elaborated. The two possible proton-transfer reaction mechanisms proposed herein based on the experimental results were investigated; moreover, another two possible reaction mechanisms involving the activation of both 2,5-dichloroaniline and trichloroacetimidate by (±)-camphorsulfonic acid via dual hydrogen-bonding activation modes were evaluated. The calculated results suggest that the reaction between 2,5-dichloroaniline and trichloroacetimidate catalyzed by (±)-camphorsulfonic acid preferentially occurs through the SN1 mechanism based on the dual hydrogen-bonding activation modes.


 Please wait while we load your content...
Please wait while we load your content...