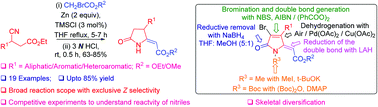
Blaise reaction: synthesis, skeletal diversification of C(4) substituted 5-ylidenepyrrol-(5H)-ones and the role of the strategically located ester on the reactivity of the nitriles†
Abstract
We have achieved a simple and convenient synthesis of several C(4) substituted 5-ylidenepyrrol-(5H)-ones from 3-aryl/heteroaryl/alkyl 3-cyanopropionates and ethyl/methyl bromoacetates via a useful variation of the classical Blaise reaction. The products were further transformed to useful small organic molecules (SOMs). Studies on the reactivity of nitriles via intra and inter-molecular competition experiments showed that anchimeric assistance from the neighbouring and strategically located ester is crucial for higher reactivity of the nitrile in the 3-cyanopropionates towards the Blaise reaction.


 Please wait while we load your content...
Please wait while we load your content...