SMM behaviour of heterometallic dinuclear CuIILnIII (Ln = Tb and Dy) complexes derived from N2O3 donor unsymmetrical ligands†
Abstract
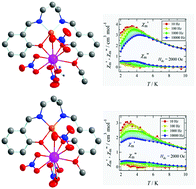
Complexes [CuL1] (1) and [CuL2] (2) of two unsymmetrical N2O3 donor ligands (where H2L1 and H2L2 are N-salicylidene-N′-3-ethoxysalicylidene-1,3-propanediamine and N-salicylidene-N′-3-methoxysalicylidene-1,3-propanediamine, respectively) have been prepared by a facile method. Using these two Cu(II) complexes as metalloligands, four new isostructural dinuclear CuIILnIII complexes [CuL(μ-NO3)Ln(NO3)2(H2O)]·CH3CN (for complexes 3 and 4, L = L1, and Ln = Tb and Dy, while for complexes 5 and 6, L = L2, and Ln = Tb and Dy, respectively) have been synthesized. In all four isomorphous complexes, the geometry around the corresponding deca-coordinated Ln(III) centres is sphenocorona, whereas the penta-coordinated Cu(II) centres are square pyramidal. The DC magnetic susceptibility measurements show the presence of ferromagnetic exchange interaction between the Cu(II) and Ln(III) centres in all four complexes. From the AC measurements, the complexes (3–6) exhibit slow magnetic relaxation properties in a DC applied field of 2000 Oe with effective energy barriers of 19.3(8), 26.1(10), 23.8(10) and 28.2(5) K, respectively.


 Please wait while we load your content...
Please wait while we load your content...