Self-supported fabrication and electrochemical water splitting study of transition-metal sulphide nanostructured electrodes†
Abstract
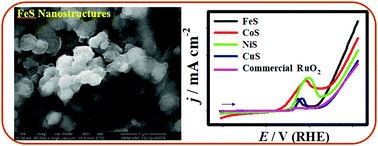
Design of efficient, low-cost and durable water splitting electrocatalyst is a central to the field of electrochemical energy conversion and storage technologies. Herein, we report a facile fabrication of a variety of nanostructured transition metal sulphides (iron sulphides (FeS), cobalt sulphides (CoS), nickel sulphides (NiS), and copper sulphides (CuS)) on a bare nickel foam (NiF) substrate via a one-step electrochemical strategy. The fabricated FeS, CoS, NiS and CuS nanostructures were effectively employed as potential electrodes for enhanced oxygen evolution reaction (OER) in presence of an alkaline electrolyte (1.0 M KOH). Our primary electrocatalytic OER study reveals that the FeS nanostructures exhibited the best activity in terms of low onset potential (∼1.51 V vs. RHE), small over-potential (η) (∼0.32 V@10 mA cm−2), and Tafel slope (∼0.069 V dec−1), and high durability in comparison to the CoS, NiS and CuS nanostructures. A unique three-dimensional sheet-like morphology, high electrochemical active surface area (ECASA), low electrochemical impedance characteristics, rapid electron-transfer kinetics, and high structural solidity enable this novel FeS nanomaterial to be a distinct and promising electrode material for the OER activity. Thus, the complete electrocatalytic OER activity of the transition metal sulphide nanostructures follows the order: FeS > CoS > NiS > CuS. Our results on the optimized design of transition metal sulphide-derived electrodes based on the chemical composition, surface morphology, abundant active sites, etc., would further improve the OER activity for water electrolysis.


 Please wait while we load your content...
Please wait while we load your content...