Influence of the ammonium salts used in the Brønsted acid catalyzed hydrothermal decomposition of d-glucose towards 5-HMF†
Abstract
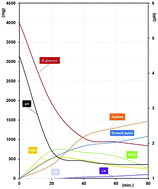
In this work, we compare the effect of two ammonium salts (ammonium sulfate and ammonium acetate) used as promoters in the hydrothermal decomposition of D-glucose with the results obtained in the presence of sulfuric acid. For the first time, we demonstrate that ammonium acetate is not a good promoter due to the low conversion of D-glucose. Conversely, ammonium sulfate appears as a much more promising promoter. Indeed, even if a large amount of humins is observed, 5-HMF is obtained in good yield with a fast conversion of D-glucose. After isolation and characterization of the liquid and solid phases, the role of ammonium sulfate was thoroughly investigated. Structural characterization of the humins obtained from D-glucose and ammonium salts is also proposed. In addition, we demonstrated that with an ammonium-containing promoter, part of the nitrogen atoms is inserted in the humin structure as pyrrole derivatives.


 Please wait while we load your content...
Please wait while we load your content...