Comparative study of ASNase immobilization on tannic acid-modified magnetic Fe3O4/SBA-15 nanoparticles to enhance stability and reusability
Abstract
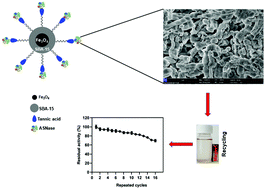
In this work, L-asparaginase was immobilized on tannic acid-modified magnetic mesoporous particles. In brief, Fe3O4/SBA-15/tannic acid magnetic particles were synthesized, and their structures and morphologies were fully characterized using various methods. The properties of the free and immobilized enzyme were examined in terms of pH, temperature, thermal stability, storage stability, and reusability. Moreover, the effects of metal ions, inhibitors and organic solvents on the activity of the immobilized enzyme were investigated. Compared to the free enzyme, the immobilized enzyme possessed better tolerance to changes in ambient temperature and pH. Additionally, thermal incubation results showed that the free enzyme lost its activity, while the immobilized enzyme exhibited the opposite behavior. Most strikingly, the immobilized L-asparaginase exhibited a high degree of activity (70%) after being reused 16 times while also demonstrating 71% and 63% storage stability of the initial activity even after 28 days at 4 °C and room temperature, respectively. Together with these results, L-asparaginase was successfully immobilized upon Fe3O4/SBA-15/tannic acid magnetic nanoparticles with improved stability properties. This support holds great potential and opens up a novel perspective for growing applications.


 Please wait while we load your content...
Please wait while we load your content...