A new synthesis methodology for SiO2 gel-based nanostructures and their application for elimination of dye pollutants
Abstract
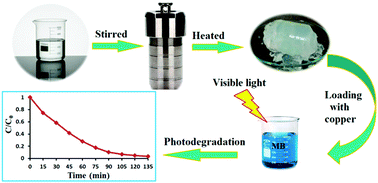
The synthesis of SiO2 gel-based nanostructures via a hydrothermal process was described in this contribution. Copper acetate was used for the preparation of an active photocatalyst based on these silica nanostructures. Two methods of post-synthesis and during-synthesis procedures were adopted for incorporation of copper in the structures. The products were characterized by X-ray diffraction (XRD), FT-IR, diffuse reflectance spectroscopy (DRS), transmission electron microscopy (TEM), field emission scanning electron microscopy (FESEM), energy dispersive spectroscopy (EDS), nitrogen adsorption–desorption analysis (BET and BJH) and TGA/DSC measurements. The BET analysis showed that the silica gel-based nanostructure possesses a specific surface area of 1336.4 m2 g−1. Methylene blue (MB) was used as a model dye pollutant in aqueous solution. The effect of pH and contact time on the adsorption of MB onto our nanostructure was studied. It was observed that 64% of MB was adsorbed in the first 3 minutes. The photocatalytic activity of the copper species/silica gel-based nanostructure was studied for MB dye degradation under visible-light irradiation. The plasmonic copper metal–silica gel-based nanostructure displayed very high photocatalytic activity. The kinetic study of the MB degradation process showed that it follows a pseudo first-order model.


 Please wait while we load your content...
Please wait while we load your content...